Hydrogen Purification
Hydrogen (H2) is considered as the fuel of future. When it is burned, it generates only water as a byproduct, meaning no harmful greenhouse gas emissions. Currently the majority of the hydrogen of the world is produced via the steam reforming and auto reforming of natural gas (mainly methane, CH4). During these process, carbon dioxide (CO2) is produced as a by-product. Currently most CO2 produced during hydrogen production is released to atmosphere without carbon capture. The hydrogen produced in these processes is often called “grey hydrogen” as it still contributes to the greenhouse gas emissions. But the steam reforming and auto reforming of natural gas can be coupled with carbon capture and storage, therefore, significantly reduces its overall greenhouse gas emission. The hydrogen produced in these processes with carbon capture and storage is often called “blue hydrogen”.
At zenith purification llc, we are developing asymmetric polymeric membranes to remove impurities such as carbon dioxide (CO2) and hydrogen sulfide (H2S) from hydrogen (H2) and syngas. Our membranes are based on facilitated transport mechanism and are highly selective to acid gases such as CO2 and H2S. The membranes have low hydrogen permeability, which means (1) high purity H2 can stay on the retentate side without loss of pressure, and (2) the H2 loss will be minimal. Our membrane is suitable for the decarbonization from the widely used steam reforming and auto reforming process to produce hydrogen. The following part will explain why our membrane is particularly suitable for hydrogen production and purification.
As shown in figure 2, in a membrane based on the solution-diffusion mechanism, all the gas molecules transfer across the membrane via (1) solution into the membrane and (2) diffusion across the membrane. A gas with higher solubility and/or with higher diffusivity (such as H2) will have better selectivity and therefore, be easier and faster to transfer across the membrane than the gas with lower solubility and/or lower diffusivity (such as CO2, N2). In a membrane primarily based on the solution-diffusion mechanism, H2 has a higher selectivity vs. Other gases such as CO2, N2, CO, in another word, the membrane selectively removes H2 from the gas mixture such as syngas of the steam reforming process. For the syngas process, using such a membrane will lead the pressure loss of H2 stream, which needs a re-pressurization step later on in the process.
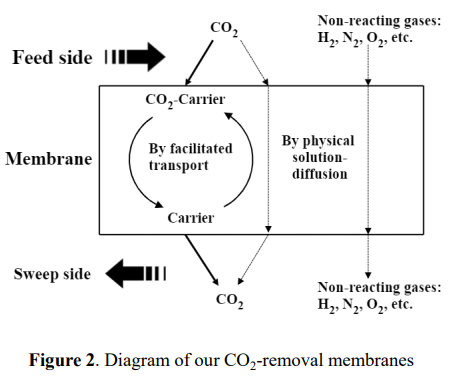
(such as CO2) on the feed side of a membrane, move across the membrane, and release this component on the downstream side. As a result, the component being facilitated is removed from the retentate side to the downstream side, and the other components (such as H2, N2), which are not affected by facilitated transport, move across the membrane at much lower flow rates, therefore, are mostly retained on the retentate side. In fixed carrier membranes, the specific component reacts at one carrier site and then hops to the next carrier site along the direction of the concentration driving force via the hopping mechanism.
Our membrane is particularly suitable for the carbon capture from the steam reforming process and auto reforming process due to the membrane’s high CO2 permeability and low H2 permeability, which offers many advantages: (1) carbon capture is effective and efficient, (2) the captured CO2
cost is low; (3) high purity H2 can stay on the retentate side without loss of pressure, and (4) the H2 loss is minimal.


