Carbon Capture
Carbon dioxide (CO2) is one of the primary greenhouse gases in Earth’s atmosphere. The CO2 concentration in atmosphere increased from 280 ppm (part per million) in 1750 at the beginning of the Industrial Revolution to 412 ppm in 2020. Increases in atmospheric CO2 are responsible for about two-thirds of the total energy imbalance that has been causing Earth’s temperature increases. Nations around the world need to make drastic reductions in CO2 emissions into the atmosphere to meet the maximum 1.5 °C temperature increase scenario recommended by the Intergovernmental Panel on Climate Change.
Industrial CO2 separation technologies can be divided into three main categories: (1) separation with sorbents/solvents; (2) cryogenic distillation process;(3) separation with membranes.
In the separation with sorbents/solvents technologies, CO2 is first adsorbed on surface of a material, such as zeolite and activated carbon, or absorbed into a bulk material, such as a solvent, an aqueous alkanolamine solution, then the CO2 -loaded sorbent/solvent is depressurized or heated to release CO2.
The most widely used sorbents/solvents technologies include pressure swing adsorption (PSA) and amine scrubbing. However, these sorbents/solvents processes all require regeneration of sorbents/solvents, which are energy-intensive and require additional chemicals (sorbent/solvent).
In the cryogenic distillation process, a gas with different components is liquified by a series of compression, cooling and expansion steps, then the liquid is distilled at the boiling points of the different components. The most widely used cryogenic distillation process is to separate oxygen (O2), nitrogen (N2), helium from air. The cryogenic distillation process is highly energy-intensive.
In the CO2 separation with membranes, a gas mixture containing CO2 enters the membrane unit on the feed side. Due to the selectivity of the membrane, CO2 is permeating at a higher flow rate than other components through the membrane to the permeate side. The membrane material can be polymeric, metallic, or ceramic. Over the last 40 years, separations using polymeric membranes have been widely adapted for environmental and energy applications in numerous industries, in particular, water purification, desalination, and more recently gas separation. Using membrane for CO2 separation provides one promising approach to capturing and concentrating CO2 with reduced energy consumption, enhanced weight and space efficiency, and operational simplicity. In general, membrane separation is considered the most energy-efficient technology for CO2 separation.
In the separation with sorbents/solvents technologies, CO2 is first adsorbed on surface of a material, such as zeolite and activated carbon, or absorbed into a bulk material, such as a solvent, an aqueous alkanolamine solution, then the CO2 -loaded sorbent/solvent is depressurized or heated to release CO2.
The most widely used sorbents/solvents technologies include pressure swing adsorption (PSA) and amine scrubbing. However, these sorbents/solvents processes all require regeneration of sorbents/solvents, which are energy-intensive and require additional chemicals (sorbent/solvent).
In the cryogenic distillation process, a gas with different components is liquified by a series of compression, cooling and expansion steps, then the liquid is distilled at the boiling points of the different components. The most widely used cryogenic distillation process is to separate oxygen (O2), nitrogen (N2), helium from air. The cryogenic distillation process is highly energy-intensive.
In the CO2 separation with membranes, a gas mixture containing CO2 enters the membrane unit on the feed side. Due to the selectivity of the membrane, CO2 is permeating at a higher flow rate than other components through the membrane to the permeate side. The membrane material can be polymeric, metallic, or ceramic. Over the last 40 years, separations using polymeric membranes have been widely adapted for environmental and energy applications in numerous industries, in particular, water purification, desalination, and more recently gas separation. Using membrane for CO2 separation provides one promising approach to capturing and concentrating CO2 with reduced energy consumption, enhanced weight and space efficiency, and operational simplicity. In general, membrane separation is considered the most energy-efficient technology for CO2 separation.
At Zenith Purification LLC, we are developing a polymeric membranes to remove CO2 from air, flue gas, biogas, and natural gas. Our membrane has an asymmetric structure. As shown in Figure 1, this membrane has two layers: (1) a non-porous selective layer; and (2) a microporous support coated on non-woven fabric to provide mechanic strength of the membrane. This membrane structure enables the membrane to have maximum flux and mechanic strength, and is still easy to manufacture. Unlike some absorption-based and adsorption-based carbon capture technologies, our membrane (1) does NOT need energy-intensive regeneration; and (2) does NOT need additional chemicals during operation.
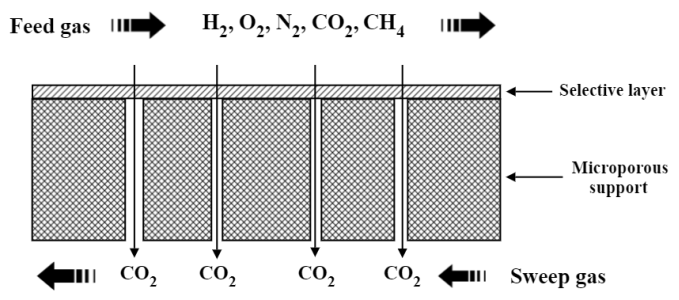
Figure 1. Schematic of our composite membrane
Our membranes can remove CO2 from gas mixtures with a wide range of CO2 concentrations. Typical CO2 concentrations (dry basis) in these gas mixtures are: 0.041% in air, 3-5% in flue gas from natural gas power plants, 12-15% in flue gas from coal-fired power plants, 18-22% in flue gas from cement plants, 30-40% in biogas, and 0-30% in raw natural gas.
Using our membrane is an effective and efficient way to capture CO2. It is worth noting that the CO2 capture cost is highly dependent on the CO2 concentrations in the gas mixtures. Typically, the higher the CO2 concentration, the lower the CO2 capture cost.
Using our membrane is an effective and efficient way to capture CO2. It is worth noting that the CO2 capture cost is highly dependent on the CO2 concentrations in the gas mixtures. Typically, the higher the CO2 concentration, the lower the CO2 capture cost.


